Chemical Equilibria
The first step in the conversion of coal into gasoline is the steam reforming reaction.
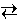 CO (g) + H2 (g)
CO (g) + H2 (g)Suppose that 0.0500 mole each of carbon, water, carbon monoxide, and hydrogen are placed in a 10.0 L container, which is then heated to 1000 K and the steam reforming reaction is allowed to reach equilibrium. Note that carbon is present as a solid. At 1000 K, water, carbon monoxide and hydrogen are all gases.
How does a change in the amount of a reactant or product added to the system affect the equilibrium amounts of reactants and products in the system?
Although one might place 0.0500 mole of water in the system, at equilibrium there will likely be a different amount of water present. As the system reacts to reach equilibrium, some compounds will be partially consumed while others will be produced.
In this experiment, for example, 0.0500 mole of each reactant and product is added to the container, but the equilibrium amounts of carbon and water are 0.0093 mole and the equilibrium amounts of carbon monoxide and hydrogen are 0.0907 mole. One consequence of the dynamic nature of chemical equilibrium is that the amount of a compound placed in a system is rarely the amount that actually exists in the system once equilibrium is achieved.
Objective
Explore the effect of changing the amounts of reactants and products placed in the system on the equilibrium composition of the system.
Experiment
The reset button is used to clear the container and add 0.0500 mole of each reactant and product to the container. For each material a slider is provided that adds or removes material from the container. The system automatically reacts to this change and reaches a new equilibrium state. The equilibrium amounts of each material are shown by the vertical bars and in the boxes on the right side of the display.
It is also possible to change the temperature and the volume of the system.
Perform the following experiments and carefully observe the behavior of the system.
- Reset the experiment. Use the H2 slider to increase the amount of hydrogen placed in the system. Observe how the equilibrium amounts of each species change. Obviously the equilibrium amount of hydrogen increases. What happens to the equilibrium amounts of carbon, water, and carbon monoxide? Decrease the amount of hydrogen and observe the results.
- Reset the experiment. Use the H2O slider to decrease the amount of water placed in the system. Observe how the equilibrium amounts of each species change.
- Reset the experiment. Use the equilibrium amounts, the system volume of 10.0 L, and the system temperature of 1000. K to calculate the equilibrium constant, KC, for the reaction. Vary the amounts of carbon monoxide and hydrogen and repeat the calculation. Do you always get the same value (within experimental variability)? (Remember to use molar concentrations in calculating KC and keep the temperature constant at 1000. K.)
Discussion
Did you find a pattern in the behavior of the system as added amounts of reactants and products were varied?
The French chemist Henri-Louis Le Châtelier summarized this behavior in what is now called
Le Châtelier's Principle:
When a stress is brought to bear on a system at equilibrium, the system will react in the direction that serves to relieve the stress.
Bottom Line: Whenever you make a change to a system at equilibrium, nature tries to undo the change. The change is not fully undone, but nature partially offsets the change.
Suppose the system is at equilibrium with 0.0500 mole initial amounts of each reactant and product. When additional hydrogen is added to the system, this constitutes a "stress" on the system in that it pushes the system away from equilibrium. To return to equilibrium, nature attempts to reduce the amount of hydrogen (that is, it tries to undo the addition of hydrogen) by having the hydrogen react with carbon monoxide to produce carbon and water. Thus when hydrogen is added to the system, the equilibrium amount of carbon monoxide decreases and the equilibrium amounts of carbon and water increase.
Initial Amount of Carbon
You may have found unusual behavior associated with carbon. To see how carbon is unusual, perform the following experiment.
- Reset the experiment. Use the carbon slider to increase the amount of carbon added to the system. What happens to the equilibrium amounts of water, carbon monoxide, and hydrogen?
- Reset the experiment. Use the carbon slider to slowly decrease the initial amount of carbon in the system. Initially the equilibrium amounts of the other species do not change. At what added amount of carbon do the equilibrium amounts of the other compounds in the reaction begin to decrease? Why does this behavior occur?
- Reset the experiment. Use the carbon slider to set the initial amount of carbon to 0.0200 mole. What is the equilibrium amount of carbon? Is it appropriate to talk about the steam reforming reaction being at equilibrium under this condition? Use the equilibrium amounts to calculate the equilibrium constant. Do you obtain the same value as previously calculated?
Why are the equilibrium amounts of water, carbon monoxide, and hydrogen unaffected by the amount of added carbon? (See below for the exception.)
Effect of Temperature
- Reset the experiment. Use the temperature slider to increase the temperature of the system. What happens to the equilibrium amounts of water and carbon? What happens to the equilibrium amounts of carbon monoxide and hydrogen?
- Increasing the temperature makes more heat available to drive the reaction. Does this reaction consume heat or produce heat? Is the reaction endothermic or exothermic?
- Find a table of standard enthalpies of formation, and calculation the standard enthalpy of reaction for the steam reforming reaction. Although your value for ΔrxnHo is likely at 298 K rather than the simulation temperature, is the sign consistent with the effect of temperature on the equilibrium amounts?
Effect of Volume
- Reset the experiment. Use the volume slider to decrease the volume of the system. What happens to the equilibrium amounts of water and carbon? What happens to the equilibrium amounts of carbon monoxide and hydrogen?
- Decreasing the volume increases the partial pressures of all gas-phase reactants and products. On which side of the equilibrium arrows are there more moles of gas-phase molecules?
Equilibrium Constant
- Above, you were asked to calculate KC for this reaction at 1000. K. At this temperature, calculate KP. The gas constant is R = 0.08206 L atm K-1 mole-1.
- How precise is KC ? How precise are the concentrations used to calculate KC ?
- Within the precision for the equilibrium constant, is the value for KC constant regardless of the initial conditions?
As a check on your calculations, you should find KC = 7.23 mole/L and KP = 0.0881 atm at 1000. K
LeChatelier.html version 3.0
© 2001, 2020, 2023 David N. Blauch